Interplay between a tuberculosis vaccine and gut microbes
Vaccines are our key defense against pathogen infections and related symptoms not only in pandemic times. For example, the prevalence of M. tuberculosis (MTB) accounts for 2 billion people, adding 1.7 million cases each year. Bacillus Calmette-Guérin (BCG), the only licensed MTB vaccine is administered to infants throughout the world and is estimated to reduce neonatal mortality by 38% in high pressure infection environments. It consists of a weakened Mycobacterium bovis strain that is not pathogenic, but induces the host immune system to combat a wider range of Mycobacterium strains including MTB.
Interestingly, it has been observed that many vaccines, especially ones based on attenuated live organisms, confer lasting protection against unrelated pathogens in addition to the original stimulus.
This phenomenon, sometimes termed trained immunity has been the subject of extensive research. One puzzling question is the high variability in how different individuals respond to the vaccine and the factors that might influence it.
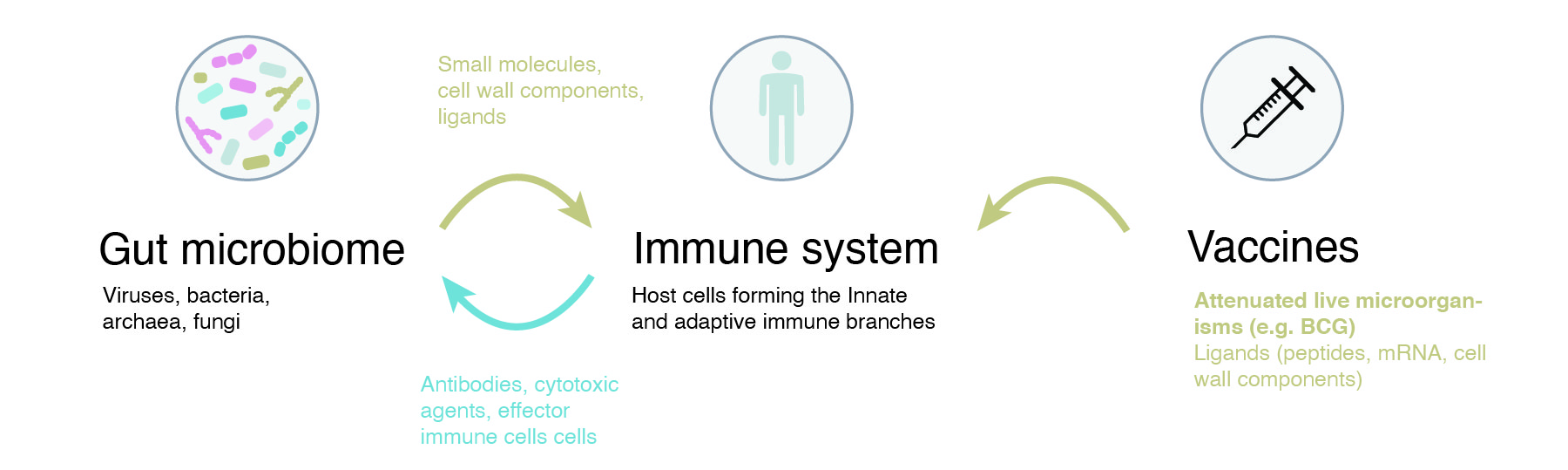
As discussed earlier, our gut microbiome plays a crucial role in training the immune system, trough production of small molecules, exposure of cell wall components or tampering with the host immune signalling. Microbiome as an ‘organ’ also reflects many aspects of our diet, environment and behaviour, so it presents a natural starting point to answer why some vaccine responses are stronger than others.
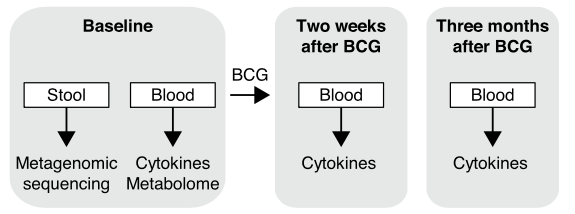
With colleague Vera P. Mourits, we investigate he influence of the gut microbiome on BCG vaccine response in a large human cohort of healthy adults. The sheer volume of data, collected at Radboud UMC in Nijmegen, Netherlands speaks to scale of such investments; 323 healthy individuals were vaccinated and followed up after two weeks and three months at which point their blood was collected and underwent a battery of different measurements.
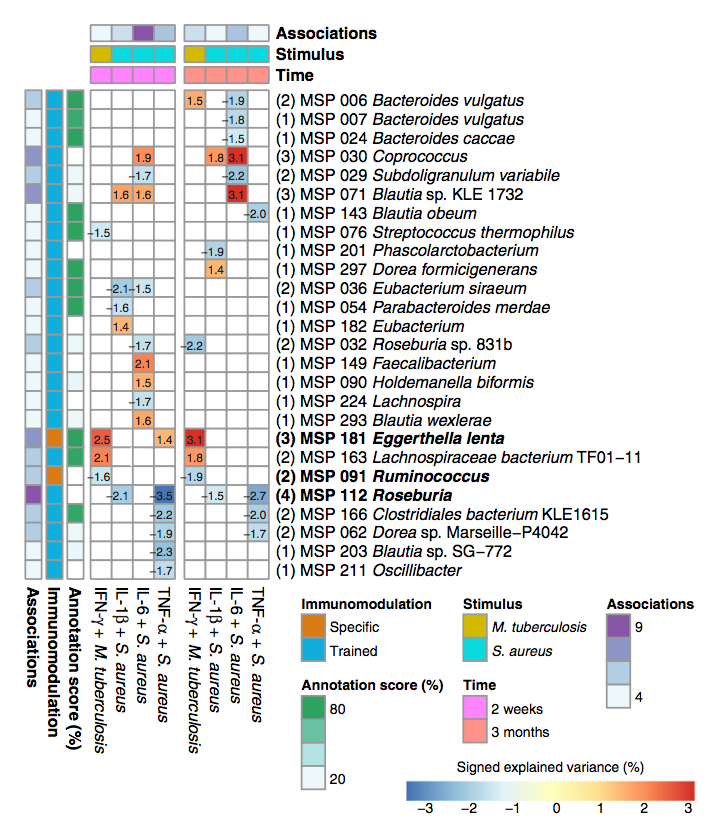
Our de novo assembly-based metagenomic analysis identified 43 potential immunomodulatory microbial species and revealed trained immune response detected by altered production of cytokines IL-6, IL-1β, and TNF-α and negatively correlates with abundance of Roseburia. We go on to identify circulating metabolites and pathways through which Roseburia species might influence host metabolism, identifying groups of enzymes in phenylalanine metabolism pathway.
The data presented here details for the first time microbiome-associated effects on BCG vaccine responsiveness in a large human cohort. The pathways and microbial species that mediate BCG-induced trained and specific immune responses contribute to the understanding of subject-specific immunity and may inform personalized strategies to enhance vaccine efficacy.
Read more in Genome Biology.