A Swiss knife for cholesterol metabolism
Non-communicable diseases are burdening the healthcare systems in much of the northern hemisphere and beyond. Cardiovascular disease can often be linked to host lipid levels, where cholesterol plays a key role in fat digestion, cell growth and signaling. Hypercholesterolemia - high cholesterol levels - is also associated with buildup of plaques and is detected in 30 - 45 % of heart attacks.
There is a years-long pursuit for potential cholesterol lowering bacteria (probiotics), which has so far yielded mixed results and left the underlying mechanisms open to interpretation. Much of the work started by noticing that consumption of fermented foods (e.g. yogurt) lead to decreased serum cholesterol levels, hinting that bacteria in those products do something useful. One mechanism that aligned with these observations was bacterial metabolism of bile acids, which triggers the host to dig in cholesterol reserves to replenish this pool. More recently, bacteria with direct cholesterol metabolizing capabilities have been found, although their abundance in humans was unknown.
Framingham Heart Study is a multi-generational patient tracking effort, which started in 1948, now in its third generation and counting. Originally designated to study the onset of cardiovascular disease, it is powered to provide detailed insights into onsets of different diseases and ailments. The latest published data included bacterial and metabolite profiles combined with diverse vitals.
With Chenhao Li and the team, we had the opportunity to delve into large-scale analyses of patient data, which revealed that intestinal cholesterol could be related to better CVD indicators, including blood glucose and triglyceride levels. These were in turn heavily associated with host gut bacterial abundances.
Among other things, we noted several microbes with the potential of passing dietary compounds (cholines, carnitines), characteristic for diets rich in red meats, down the trimethylamine-N-oxide (TMAO) pathway, a known cause of atherosclerosis and thrombosis.
Combined with screening of metabolite profiles allowed us to seek bacteria which may be related to reduced cholesterol levels.
As there may be many reasons for decreasing cholesterol levels, the high “statistical power” (size of the cohort) promises robust identification of causal factors. Indeed, we found Eubacterium, a low abundant bacterium known to convert cholesterol to products that are easier to excrete from the system, as shown in a previous study from our group. The stand out bacteria though were Oscillibacter, which had 26 representative species and was present at very high levels, reaching up to 1 in 100 bacteria in some individuals. This presented promising early evidence that Oscillibacter might have to do with decreased cholesterol levels.
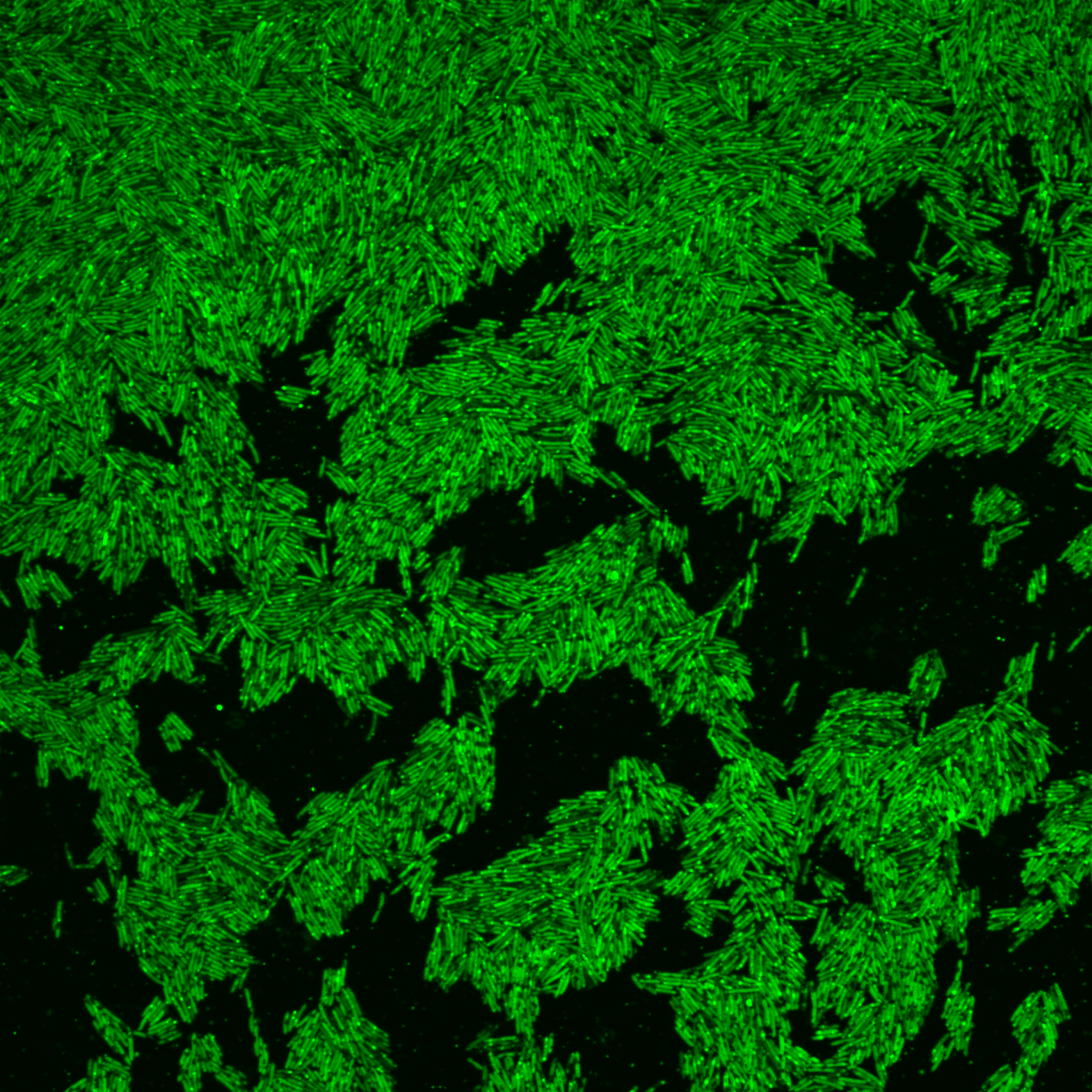
Digging through the freezers containing hundreds of bacteria in our isolate collection, early experiments showed that Oscillibacter can assimilate fluorescent cholesterol, which can be seen shining within the bacteria on the image above.
To shed even more light on the mechanisms for cholesterol metabolism in Oscillibacter, we turned to recent artificial intelligence methods, which have recently redefined the protein search field. As microorganisms evolve, they chop and change proteins so fast that they may become unrecognizable by amino acid sequence comparison methods. Rather, searching for retained protein structures is more adept at identification of conserved functions. Searching for several ways cholesterol can be metabolized, we identified several, but not all enzymes in the cholesterol excretion pathway. Interestingly, when Oscillibacter was found together with Eubacterium, the association with lower cholesterol was even stronger, hinting at potential cooperative effects.
Oscillibacter turned out to have more in its locker, including modifying cholesterol by decorating it with sugars, a mechanism that stomach bacteria use to survive in that hostile environment.
Narrowing down from modern metagenomics approaches, we also resorted to more classical bacterial isolation and culturing from patient samples. DNA sequencing confirmed that gut isolates from this exact cohort possess the predicted tools for cholesterol metabolism. This effort also enabled us to perform culture metabolomics, showing that the predicted cholesterol derivatives indeed appear when bacteria are grown in a dish.
Our journey down the cholesterol pathways in the company of Oscillibacter is not over yet. Animal model work is required to establish therapeutic potential of these bacteria or their enzymes, with careful monitoring of colonization efficiency, gene expression and circulating cholesterol levels. Dietary context also matters a lot. As the contemporary studies feed us with vast amounts of precise information, this is an exciting time to act on bacterial fat digestion.
Learn more in Cell.