Bacterial trips and messages from mothers to newborns
Humans provide room and board for thousands of bacterial species, most of them renting the space in our guts. In exchange for a notable portion of our food intake, they can help us metabolize hard-to-digest nutrients and keep our immune systems in check. Our relationship with these guests is established immediately at birth (and perhaps before), when some of the maternal bacteria relocate into their newborn host.
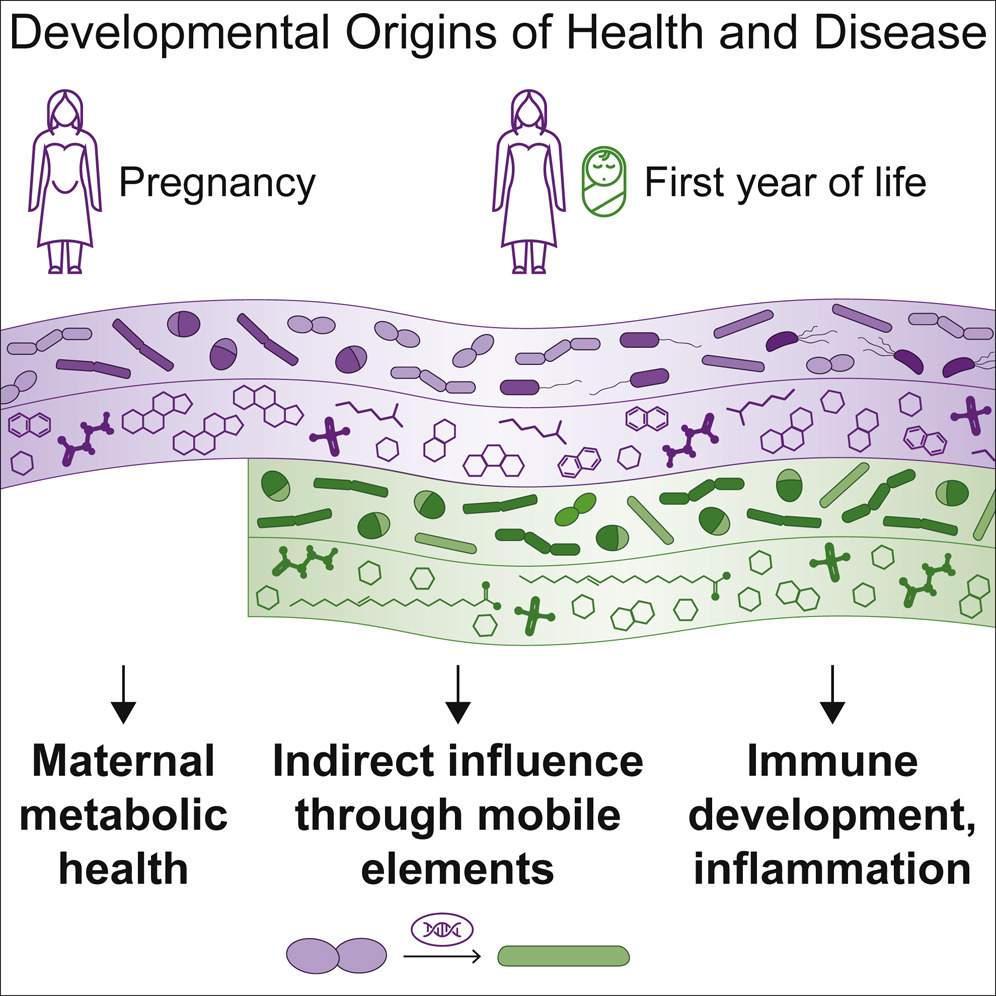
In our latest study, led by Tommi Vatanen and Eva Karolina Sjöberg Jabbar, we investigated which bacteria make the journey, their dietary preferences and the chemical compounds they produce. In a collaboration including institutes and hospitals from Massachusetts, USA and Helsinki, Finland, 70 mother/baby pairs were tracked for months before and after birth, measuring the bacteria and compounds (metabolites) they both harbor.
The infants start with a “blank slate” and rapidly accumulate bacteria, as evidenced by increased diversity and the number of different compounds increasing within the first 3 months of life. Levels of Eubacterium, Roseburia, and Blautia correlate between mothers and their infants samples, suggesting that these are the species likely to translocate. How the actual migration happens is unclear and likely specific to individuals. Primarily, co-housed household members tend to share similar gut bacteria, but direct transfer through the placenta remains highly controversial.
The data revealed a striking phenomenon where maternal bacteria share individual genes with infant microbes, rather than relocating themselves. A robust statistical analysis has shown that one can find 100% identical genes shared between species A in the mother and some different, but related, species B in the baby. Requiring strict identity between the two gene copies and the low likelihood of finding such matches by chance (in unrelated mother/baby pairs) corroborated this conclusion.
A very generous bacteria was Bacteroides cellulosilyticus, a prominent utilizer of carbohydrates in breast milk which provided many genes to infant microbes from the same microbial family. The ability to survive on milk sugars was one of the most transferred functions, highlighting the importance of adaptation in this dietary-restricted period. How and where this gene transfer happens is still an open question, but evidence suggests multiple genetic editing mechanisms in bacteria as well as the existence of “mediator” bacteria or viruses (phages).
It is intriguing to think whether bacterial families help “one of their own” to survive in a very different and resource limited environment. This is seen by an increased number of carbohydrate/microbe correlations after birth, a trend which declines as the baby ages and she or her microbial community gets less dependent on any one member.
Important implications of this study were marked differences affected by breast milk and infant formula use, which directly affected bacterial and metabolite compositions. Microbiomes in Westerners are more different than traditional populations to begin with, an effect which is probably exacerbated by increased formula use in recent years. Since our relationship with microbes has evolved over millions of years, abrupt changes in dietary practices affect the lineup of bacteria and their compounds our immune system gets to see. Indeed, diet based on breast milk and different types of formula affected chemical composition as well as immunological profiles of the infant blood. These data are aligned with an unfortunate increase in autoimmune disease with infant formula use, noted in previous reports.
Medical and dietary patterns during pregnancy birth have important implications not only on infants themselves, but also their microbial community. The latter is an important factor during the first three years of life, a time when our immune system undergoes a crucial educational phase.
Read more in Cell.