Discovery of novel bacterial clades in healthy infant development

Human breast milk, sometimes called the ultimate superfood, is a key source of nutrients not only for the growing infant but also for its developing microbiome. Indeed, human milk oligosaccharides (HMOs) are complex sugars indigestible by human enzymes and cater primarily to our bacterial friends. Therefore, breastfeeding is important to ensure stable and healthy microbial communities that contribute to digestion and immune system development.
Although increasingly rare in modern societies, the World Health Organization recommends breastfeeding for up to two years of age. It is therefore important to understand how birth and feeding practices (food, delivery modes) affect the infant gut microbiome and it’s molecular repertoire in order to back up such recommendations with data. In a study featured in Cell, lead by Tommi Vatanen, Qi Yan Ang, and Lea Siegwald, we analyze a large, longitudinal cohort of more than 200 infants from Bangladesh, sampled for multiple times points and up to two years after birth. As early months are exclusively fueled by breastfeeding, the microbes are dominated by species from Bifidobacteria. Introduction of solid foods is a major milestone in development, leading to the bloom of another genus Prevotella, commonly associated with plant based diets.
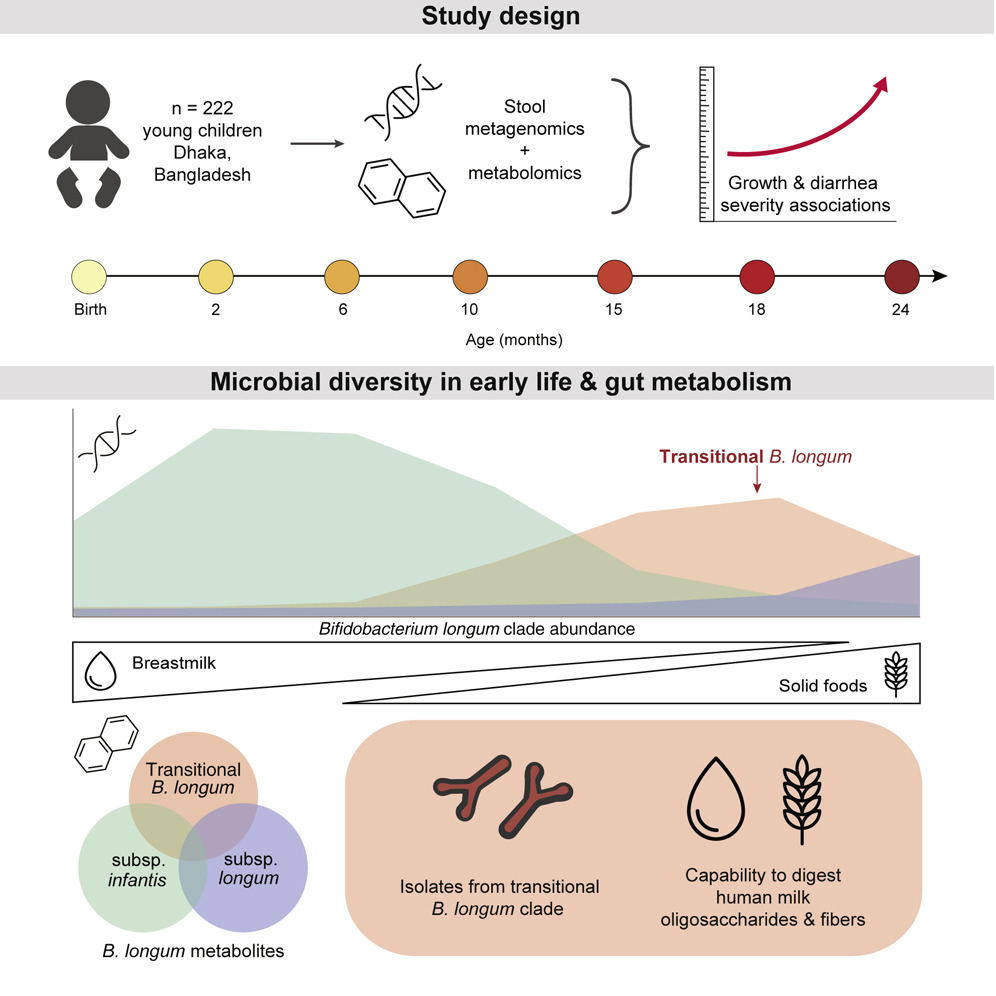
Bifidobacteria play important roles in digestion of carbohydrates as well as immune system development by producing molecules such as indolelactate. Here, we precisely characterize subspecies of a common bacteria Bifidobacterium longum. The period immediately after weaning was dominated by B. longum infantis and the transition to solid foods lead to an increase in B. longum longum. The transition between these two large communities happens through a newly discovered clade, named B. longum transitional. Comparison of the strains in three different ways (mutational analysis, metagenomics-assembled genomes and culturing isolates) confirms that this three-clade structure always appears.
The genomes of B. longum transitional harbors the capabilities to digest both human milk oligosaccharides (breastfeeding) as well as other complex glycans associated with plant based diets, including wheat and legumes. The analyses provide a clear example of how subspecies adaptation to changing environmental regimes happens at the gene level and leads to selection of best adapted subspecies.
B. longum transitional also associates with increased levels on imidazole propionic acid, which promotes neurodevelopment and pipecolic acid, a building block for many important secondary metabolites such as immunosuppressants, which is predicted to be produced from dihydrodipicolinate synthase enzyme found in this microbe. The rich trove of molecular data generated in this study, including more that 2,000 paired metabolomics and metagenomics samples promise further discoveries of important microbially-produced metabolites.
In summary, recommendations on birth practices have direct implications for healthy development of our gut microbiota, as underlined by two additional analyses in the study, showing how microbial compositions and levels of their compounds (N-acetylglutamate, indolelactate) predict diarrheal episodes and have marked effect on infant growth. The findings presented here are also an important resource in probiotic and prebiotic design to promote or recover microbial communities that work for us.
Read more in Cell.